Research
Let's do the best research in the world!
Nucleation and growth of nanometer sized particles is my most interest research field. I want to elucidate the initial stages of materials formation in molecular level incorporating the specific physical properties and singular phenomena appearing in nano-scale. I also want to know how cosmic dust particles and complex organic molecules form in the universe and how living organisms control polymorphs based on transmission electron microscopy, interferometry and microgravity experiments.
- Smoke experiment;Homogeneous nucleation from vapor phase
- Significant properties of nanoparticles:Liquid like solid
- In-situ TEM observation
- Duplicate cosmic dust in lab
- Reproduction experiment of astronomical infrared features
- Analysis of extraterrestrial minerals
- Production of carbyne crystal
- Production of functional nanoparticles
Smoke experiment;Homogeneous nucleation from vapor phase
What is smoke?

When a material evaporates in an inert gas, the evaporated hot vapor subsequently cools and nucleates to form nanoparticles that flow along with the convection current generated by the hot evaporation source. The flow resembles the smoke from a cigarette. Inside the chamber, because there is no substrate near the evaporation source, the particles nucleate homogeneously. The presence of an inert gas decreases the mean free path of the evaporant.
Reference
- Y. Kimura, K. Tsukamoto, Homogeneous Nucleation of Smoke Particles and Its Relationship with Cosmic Dust Particles (2017) 339-351. In New perspectives on mineral nucleation and growth: From solution precursors to solid materials, Eds. A. E. S. van Driessche, M. Kellermeier, L. G. Benning, D. Gebauer, Springer.
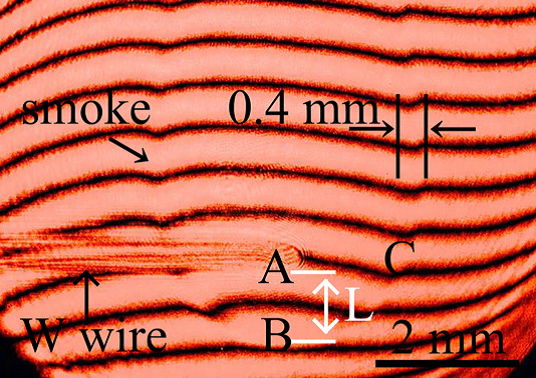
Determination of surface free energy and sticking probability
We succeed to evaluate nucleation theories based on homogeneous nucleation experiment in vapor phase and recognized that the semi-phenomenological nucleation model can be attempted to formation of nanoparticles via homogeneous nucleation. We concluded that our experiment with optical interferometric technique can be determined surface free energy and sticking probability at the supercooling condition. These two parameters are fundamental and quite necessary for investigations in various fields.
Reference
- Y. Kimura, K. K. Tanaka, H. Miura, K. Tsukamoto, Direct observation of the homogeneous nucleation of manganese in the vapor phase and determination of surface free energy and sticking coefficient, Crystal Growth & Design, 12 (2012) 3278–3284.
Homogeneous nucleation at ultra-high supersaturation condition
To investigate homogeneous nucleation and obtained quantitative data, smoke generator was newly constructed and interferometric technique was attempted for the fist time to the gas evaporation method. Finally, we achieved to obtained degree of supersaturation for homogeneous nucleation, sticking coefficient, surface free energy etc by non-contact method.
Reference
- Y. Kimura, H. Miura, K. Tsukamoto, C. Li, T. Maki, Interferometric in-situ observation during nucleation and growth of WO3 nanocrystals in vapor phase, Journal of Crystal Growth, 316 (2011) 196-200.
Oriented attachment growth in vapor phase
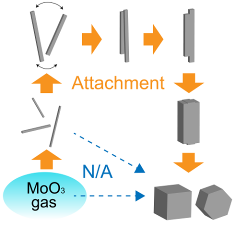
We experimentally demonstrated a growth process of MoO3 nanocrystals via oriented attachment in vapor using originally established in-situ IR technique for observing the crystalline structure and morphology of the growing nanoparticles with a combination of ex-situ TEM observations of the products. The just-nucleated MoO3 nanoparticles grow by an anisotropic monomer-by-monomer process, forming needle-shaped particles. The needle-shaped particles are selectively oriented in the gas current, leading formation of cubic. We found that the needle-shaped particles behave like macromolecular growth units for building the cubic particles.
Reference
- S. Ishizuka, Y. Kimura,* S. Yokoi, T. Yamazaki, R. Sato, T. Hama, Self-Assembly of MoO3 Needles in Gas Current for Cubic Formation Pathway, Nanoscale, 9 (2017) 10109-10116.
Two step nucleation in vapor phase
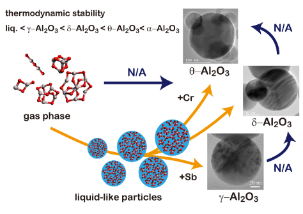
We succeed in controlling polymorphism of alumina nanoparticles (δ-, θ-, or γ-Al2O3) by adding a trace amount of metal (antimony or chromium) vapor. These crystalline phases were always directly formed from a liquid phase but not formed by solid-solid phase transition. Although the experimental condition is considerably favorable for single step crystalline nucleation, only two-step process is available. Our experimental results provide universal and detailed picture of homogeneous nucleation.
Reference
- S. Ishizuka, Y. Kimura, T. Yamazaki, T. Hama, N. Watanabe, A. Kouchi, Two-step Process in Homogeneous Nucleation of Alumina in Supersaturated Vapor, Chemistry of Materials, 28 (23) (2016) 8732–8741.
Significant properties of nanoparticles:Liquid like solid
Growth process of nanoparticles

In situ bright-field TEM observations of an example of the fusion growth. Two gold particles migrate on the surface of a silica particle seen in the bottom right-hand corner in the movie. Once the two particles contact, they fuse together to decrease their total surface energy. The lattice fringes of the two gold nanoparticles indicate that the particles are crystalline.
Reference
- Y. Kimura, K. Tsukamoto, Homogeneous Nucleation of Smoke Particles and Its Relationship with Cosmic Dust Particles (2017) 339-351. In New perspectives on mineral nucleation and growth: From solution precursors to solid materials, Eds. A. E. S. van Driessche, M. Kellermeier, L. G. Benning, D. Gebauer, Springer.
Spontaneous mixing of ionic crystals
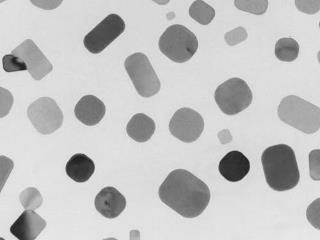
One of most famous significant properties is a spontaneous mixing of metal nanoparticles. We found similar phenomena for ionic crystal of alkali halides nanoparticles. When the ionic radii of cation and anion is closer, two different kinds of alkali halide nanocrystals diffuses each other and make a single solid-solution crystal.
Reference
- Y. Kimura, Y. Saito, T. Nakada, C. Kaito, Spontaneous Mixing of Binary Alkali Halide Crystal by Successive Evaporation, Physica E: Low-dimensional Systems and Nanostructures, 13 (2002) 11-23.
- Y. Kimura, Y. Saito, T. Nakada, C. Kaito, Spontaneous Alkali Halide Formation by the Use of KBr-KCl System, Phys. Low-Dim. Struct., 1/2 (2000) L1-L7.
In-situ TEM observation
Clarified a part of the defect formation mechanisms in a protein crystal
The production of high quality protein crystals with few defects directly leads to the efficiency of drug design. We succeefuly observed defects introduced in the early stages of protein crystallization using a transmission electron microscope. Based on the defect density with the growth, it was found that the origin of the defects is in the early stages of crystallization just before and after the nucleation. This result implies the nucleation process is more crucial than the growth process for improving crystal quality. This is also an important clue in improving the quality of other functional crystals.
This study made the cover of Soft Matter.
Reference
- T. Yamazaki, A. E. S. Van Driessche, Y. Kimura, High mobility of lattice molecules and defects during the early stage of protein crystallization, Soft Matter, 16 (2020) 1955-1960.
Two different amorphous particles activate protein crystal nucleation
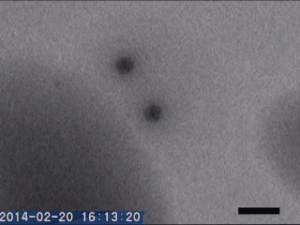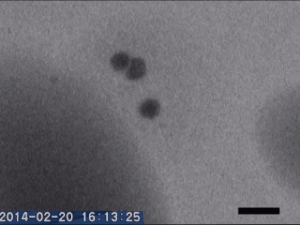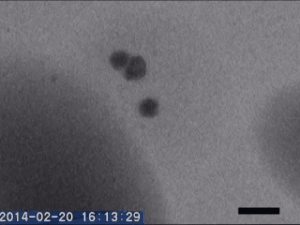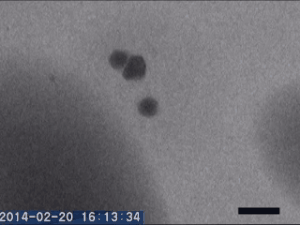
Crystal nucleation and growth represent core concepts in disciplines ranging from climate science to the pharmaceutical industry. Although the processes that drive crystal growth have been thoroughly examined, nucleation remains poorly understood and is the area where careful experimental measurements diverge from advanced theory. Focusing on proteins, Tomoya Yamazaki et al. observed lysozyme crystals nucleating in situ using time-resolved liquid-cell TEM, a technique which produces nanoscale images of samples sandwiched within nanofabricated liquid cells. The images reveal that protein-rich mesoscopic-scale particles, previously argued to be dense liquid precursors to nucleation, do not coalesce even when in contact with one another. The authors show that these particles are amorphous solid acting as heterogeneous nucleation sites for a second non-crystalline particle, likely dense liquid, which appears only less than a few seconds before the onset of crystal nucleation. As such, the authors argue, lysozyme crystallization is facilitated by two different types of amorphous particles performing different roles. Although the results require further study, the findings depart significantly from current theories about how crystals nucleate and demonstrate that recent technical advances in nanoscale fabrication and TEM can help better understand crystal nucleation more broadly.
Reference
- T. Yamazaki, Y. Kimura, P. G. Vekilov, E. Furukawa, M. Shirai, H. Matsumoto, A. E. S. Van Driessche, K. Tsukamoto, Two types of amorphous protein particles facilitate crystal nucleation, Proceedings of the National Academy of Sciences of the United States of America, 114 (2017) 2154-2159.
Direct observation of clustering process in nanoscale

We present for the first time a “live show” of the early stages of nucleation. You can enjoy that spectacle in a video that was recorded by TEM with a resolution of 0.75 nm showing the formation and dissolution of pre-critical nanoclusters from a solution. The recording of the birth and death of these nanocrystals was possible thank to a novel technique we have devised to avoid solvent evaporation. To that aim we used an ionic liquid. We looked the process during dissolution process by heating. We dissolved previously formed nanocrystals while observing them by TEM, thus searching within a narrow area surrounding the dissolving crystals where concentration was very close to equilibrium. With this approach, we have observed for the first time the formation of new pre-critical clusters within a saturated solution. Our findings in the understanding of how crystals form will have many implications in the actual control of the crystallization of nanoparticles and nanomaterials. We have also discovered that dissolution at the nanoscale takes place through mass fluctuations and not by a smooth and continuous loss of mass.
Reference
- Y. Kimura, H. Niinomi, K. Tsukamoto, J., M. García-Ruiz, In Situ Live Observation of Nucleation and Dissolution of Sodium Chlorate Nanoparticles by Transmission Electron Microscopy, Journal of the American Chemical Society, 136 (2014) 1762-1765.
Sublimation process on a crystal surface: Pb (111)

Sublimation process can be considered as a reverse process of crystal growth by a single step flow. Against the classical view, the Pb nanocrystal was sublimated by two or four planes simultaneously.
Reference
- T. Tanigaki, H. Suzuki, Y. Kimura, Y. Saito, C. Kaito, Atomic observation of the sublimation process of the Pb (111) surface, Surface Review and Letters, 10/2-3 (2003) 455-459.
Sublimation process on a crystal surface: PbTe (100)
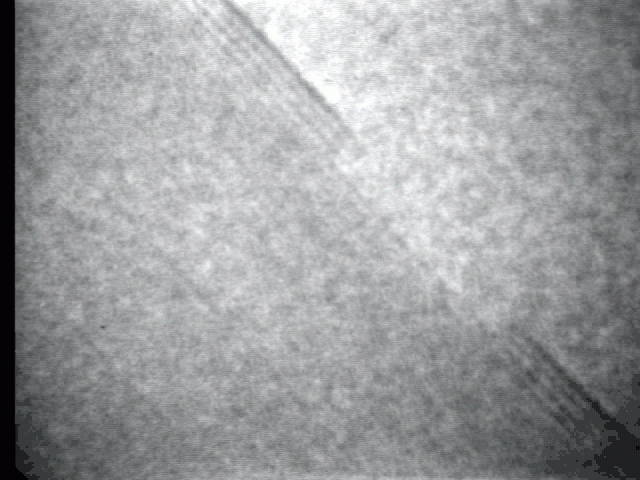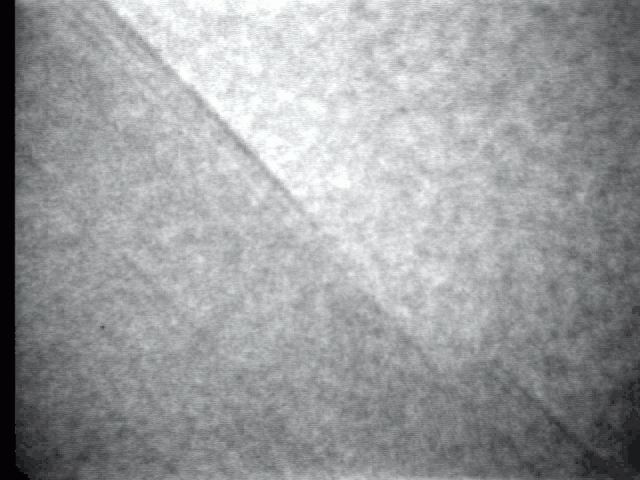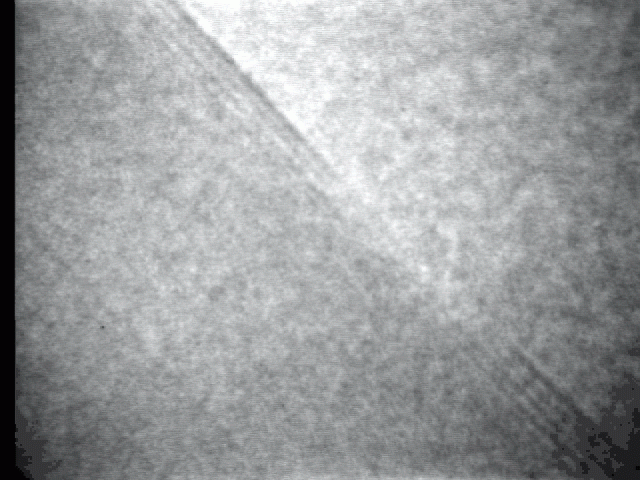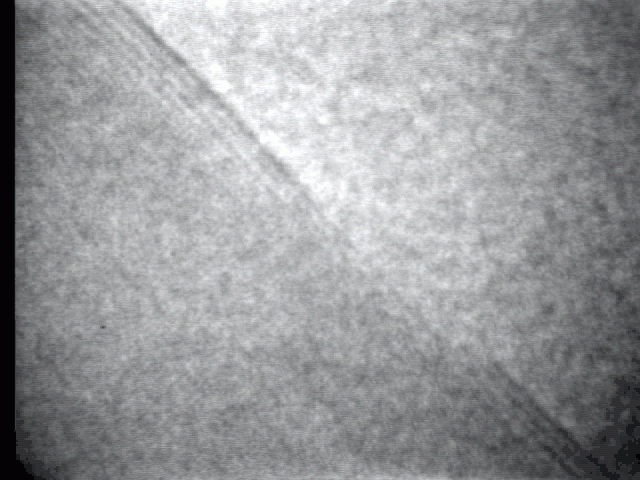
Sublimation process of semiconductor nanocrystal was observed directly at high-temperature by TEM.
Dissolution of atoms into a crystal: Carbon on a SiC nanoparticle
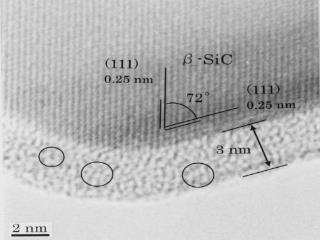
Diffusion of carbon layer into a SiC nano-crystal was directly observed by TEM with atomic-resolution TEM at 800 oC.
Reference
- Y. Kimura, Y. Saito, C. Kaito, High-temperature behavior of an amorphous carbon layer on SiC particles, Surface Science Letters, 527/1-3 (2003) L219-L221.
Duplicate cosmic dust in lab
Pure iron grain is rare in the universe

Where in the universe is iron (Fe)? This is one of the greatest mysteries in astronomy (known as so-called “missing-iron problem”). Our microgravity experiments achieved to mimic formation process of Fe grains in the gas ejecta of evolved stars. As a result, we successfully determined the sticking probability of gaseous atoms onto clusters is only ~0.002%, which is much lower than the conventional value of 100%. This suggests that the formation of Fe grains is extremely ineffective in astronomical environments. Accordingly, our result argues against the hypothesis that most of Fe exists as pure metal in the universe, and poses a challenging problem to the astronomical community in terms of the whereabouts of cosmic Fe.
Reference
- Y. Kimura,* K. K. Tanaka, T. Nozawa, S. Takeuchi, Y. Inatomi, Pure iron grains are rare in the universe, Science Advances, 3 (2017) e1601992.
Possible new scenario of silicate formation

We performed a condensation experiments, which correspond to the condensation of cosmic dust, using a mixture gas of hydrogen, oxygen, silicon and magnesium. Against with our prediction, magnesium siliside (Mg2Si) grains were produced instead of forsterite (Mg2SiO4), which is most usual and abundant solid state inorganic mineral in space. In view of our experimental results, several astronomical mysteries will be explained; why the composition of the precursor of crystalline forsterite was the composition of Mg:Si=2:1, why the founding amount of presolar forsterite is so less, why the composition of crystalline olivine is iron poor and why crystalline silicates are only produced around evolved stars with higher mass loss rate. Therefore, we will provide a completely new scenario concerning material evolution of silicate not only in protosolar nebula but also around evolved stars.
Reference
- Y. Kimura, J. A. Nuth III, A Seed of Solar Forsterite and Possible new Evolutional Scenario of Cosmic Silicates, The Astrophysical Journal Letters, 697 (2009) L10-13.
Silicate dust with IR signature of partial crystallization
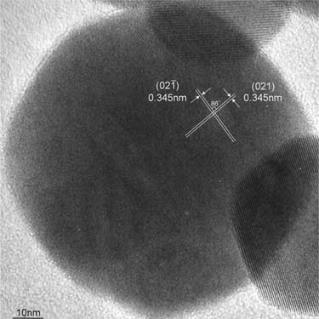
Infrared signature originated partially crystallized silicate particles around AGB stars were successfully reproduced by laboratory experiment. Almost all of the infrared features caused by silicate show a mixture of crystalline and amorphous in astronomical observation. Up to now, infrared spectra with the absorption feature by the mixture of crystalline and amorphous were only duplicated by an annealing of previously produced amorphous silicate. The production of partially crystallized silicate by condensation experiment argues that a new explanation of the existence of silicate with both phases of crystalline and amorphous around evolved stars.
Reference
- Y. Kimura, S. Sasaki, H. Suzuki, A. Kumamoto, M. Saito, C. Kaito, Experimental Demonstration of Condensation of Mg-Bearing Silicate Grains around Evolved Stars, The Astrophysical Journal, 684 (2008) 1496-1501.
Trigger for dust formation
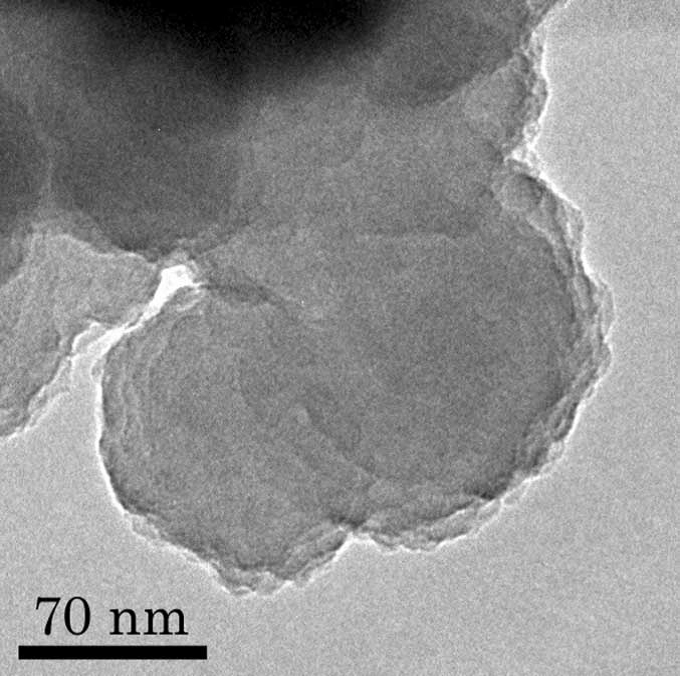
Our motivation on the study is “What is the driving force (trigger) to form cosmic dusts?". This paper suggests that the possibility and importance of other driving forces to form cosmic dusts except for condensation as gas cools. During the laboratory experiment, we found that plasma and ultraviolet radiation make silicate smoke particles with different character compared with the particles formed by combustion, from hydrogen, silane, iron pentacarbonyl and oxidant gases. For example, the silica particles produced contained hydro-silicate, which was produced directly from gas phase and showed significant infrared feature. This study becomes a trigger of a new discussion concerning grain formation in many kinds of different environments.
Reference
- Y. Kimura, J. A. Nuth III, What is the Driving Force to Form Refractory Oxide Grains? Silicate Spectra Depend on their Formation Environment, The Astrophysical Journal, 664 (2007) 1253-1263.
Core-mantle grain
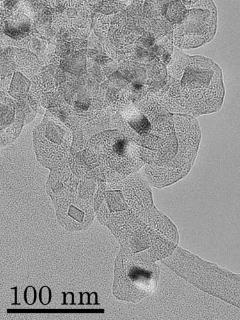
We demonstrated formation of analogs of TiC-core, graphitic-mantle spherules founding in meteorites using a new production method of carbon grain; Boudouard reaction(2CO → CO2 + C). Our experimental result would be provided new constraints of a formation condition of grains around AGB stars.
Reference
- Y. Kimura, J. A. Nuth III, F. T. Ferguson, Formation of TiC-core, graphitic-mantle grains from CO gas, Meteoritics & Planetary Science, 41 Nr 5 (2006) 673-680.
Formation of nanoparticles with non-mass dependent isotope fractionation
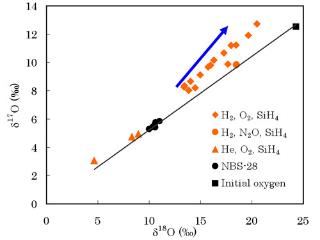
We reported the first production of non-mass dependently fractionated silicate smokes from the gas phase at room temperature from a stream of silane and/or pentacarbonyl iron in a molecular hydrogen (or helium) flow mixed with molecular oxygen (or nitrous oxide). The smokes were collected from the surfaces of the copper electrodes after each experiment. We hypothesize at least two types of fractionation processes occurred during formation of the solids; a mass dependent process that made isotopically lighter oxides compared to our initial oxygen gas composition followed by a mass independent process that produced oxides enriched in 17O and 18O. The maximum D17O observed is +4.7 ‰ for an iron oxide produced in flowing hydrogen, using O2 as the oxidant. More typical displacements are 1 – 2 ‰ above the equilibrium fractionation line. The chemical reaction mechanisms that yield these smokes are still under investigation.
Reference
- Y. Kimura, J. A. Nuth III, S. Chakraborty, M. H. Thiemens, Non-Mass-Dependent Oxygen Isotopic Fractionation in Smokes Produced in an Electrical Discharge, Meteoritics & Planetary Science, 42 (2007) 1429-1439.
Reproduction experiment of astronomical infrared features
Reproduction of 13 micron band under microgravity condition using a sounding rocket
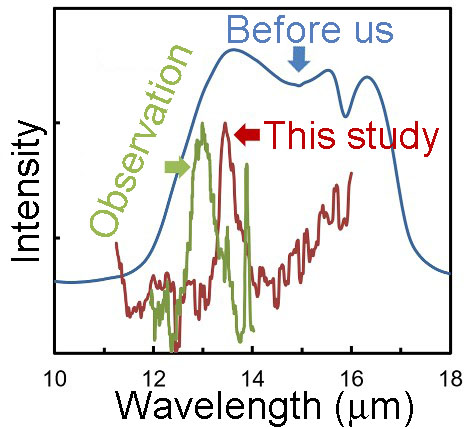
Objective of this study is make a constrain on oxygen dust formation in a gas out flow from evolved stars. Using a sounding rocket, formation of alumina dust around O-rich late-type stars and its 13 micron band was successfully duplicated by a specially designed experimental system. This finding provides a solid basis for elaborating models of condensation of dust around oxygen-rich evolved stars.
Reference
- S. Ishizuka, Y. Kimura, I. Sakon, H. Kimura, T. Yamazaki, S. Takeuchi, Y. Inatomi, Sounding-rocket microgravity experiments on alumina dust, Nature Communications, 9 (2018) 3820.
In-situ measurements of IR spectra
We developed a new experimental method for direct observation of infrared spectra of dust analogues during nucleation and growth in laboratory. As the result, free-flying dust analogues show infrared spectra with shift of peak positions and narrow band width compared with that obtained by conventional method using potassium bromine and its well agree with the theoretical estimation.
References
- S. Ishizuka, Y. Kimura, T. Yamazaki, In Situ FT-IR Study on the Homogeneous Nucleation of Nanoparticles of Titanium Oxides from Highly Supersaturated Vapor, Journal of Crystal Growth, 450 (15) (2016) 168-173.
- S. Ishizuka, Y. Kimura, I. Sakon, In- situ infrared measurements of free-flying silicate during condensation in the laboratory, The Astrophysical Journal, 803 (2015) 88 (6pp).
Successfully duplicated IR feature of astronomical silicate
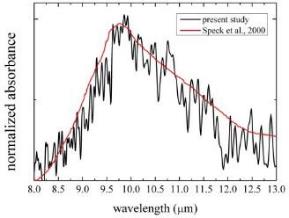
We applied a method for direct observation of infrared spectra of dust analogues to elucidate a formation process of magnesium silicate and succeeded reproduction of the spectrum of astronomical silicate for the first time. In the experiment, the initial condensate of Mg-bearing silicate particles is a melt droplet at least during the initial stages of nucleation and growth. We do not claim that the astronomical silicate is a melt of silicate, but most probably amorphous silicate with a similar structure of its melt. Later on, the melt silicate crystalizes from a supercooled droplet. This experimental result gives us a new scenario to explain the presence of low-temperature crystalline silicates around evolved stars, which cannot be explained by current proposals such as direct condensation or by annealing of amorphous silicate accompanying high-temperature processes.
Reference
- S. Ishizuka, Y. Kimura, I. Sakon, In- situ infrared measurements of free-flying silicate during condensation in the laboratory, The Astrophysical Journal, 803 (2015) 88 (6pp).
Origin of 6.8μm feature of a young stellar object
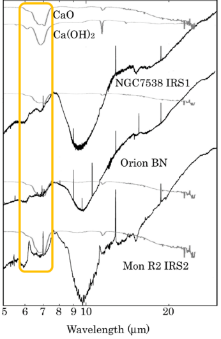
CaO and Ca(OH)2 are new excellent candidates to explain 6.8 mm feature, which is observed in young stellar objects. The production of CaO and Ca(OH)2 grains and observation of their infrared spectra were carried out in our laboratory. We note that CaO-rich grains are seen in all meteoritic CAIs and the 6.8 mm feature has only been observed in young stellar objects. Therefore, we consider CaO grains to be a plausible candidate to explain the 6.8 mm feature and hypothesize that they are produced in the hot interiors of young stellar environments.
Reference
- Y. Kimura, J. A. Nuth III, Laboratory synthesized calcium oxide and calcium hydroxide grains: A candidate to explain the 6.8 μm band, The Astrophysical Journal, 630 (2005) 637-641.
Fullerene in the universe
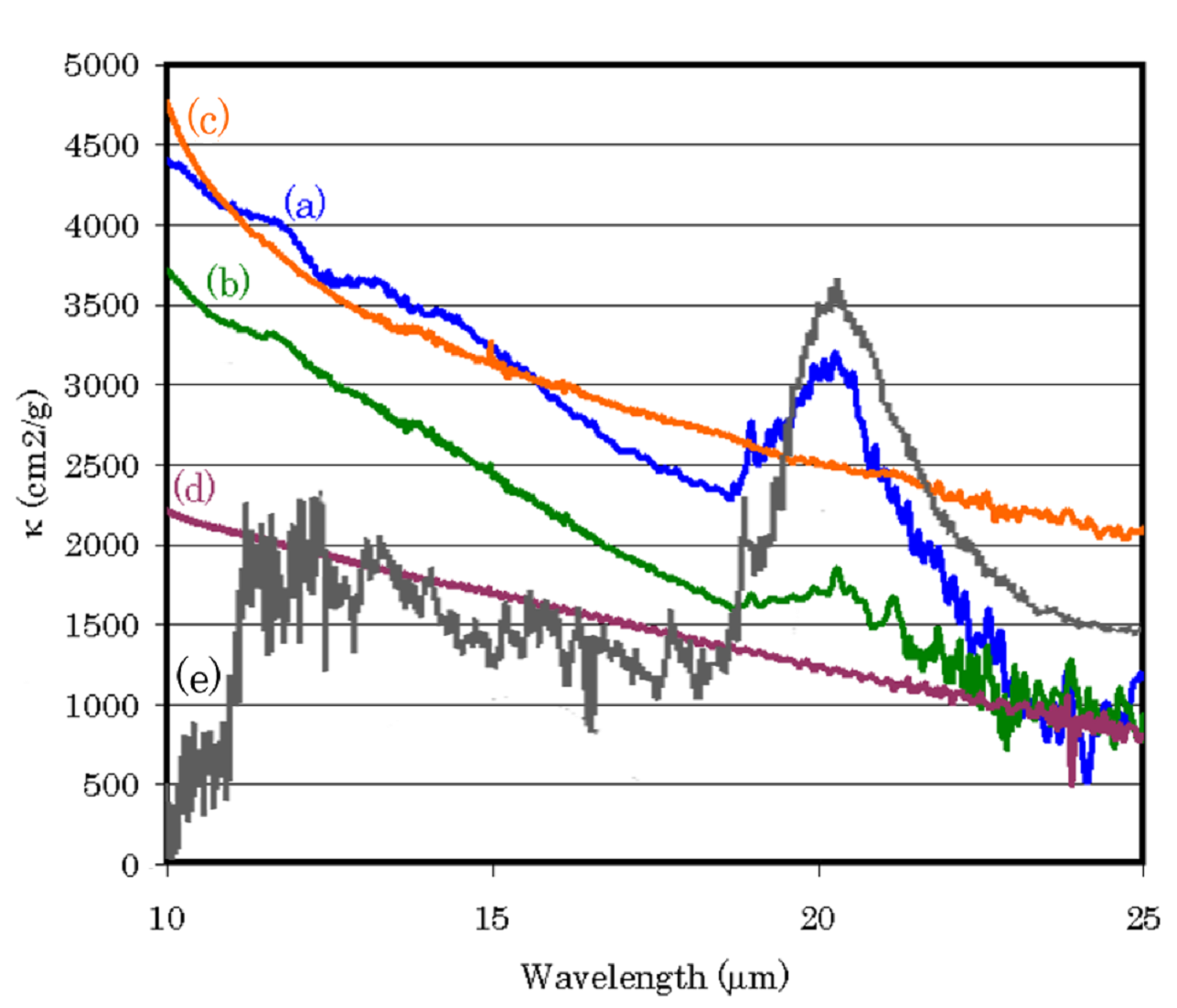
We produced large cage carbon particles, such as large fullerenes, with Ti atoms and measured their infrared spectra. As a result, a 21-micron feature, which is most enigmatic feature of carbon star, was perfectly coincident with the stellar feature. Furthermore, the small but significant fullerene peak at 19.0 micron of our samples is perfectly coincident with the peak at 19 micron observed in the post-AGB star. Therefore, we propose that fullerenes with Ti atoms are most plausible and noble candidate of 21-micron feature of post-AGB stars. This result also implies a formation of fullerenes in the stellar wind.
Reference
- Y. Kimura, Joseph A. Nuth III, Frank T. Ferguson, Is the 21-μm feature observed in some post-AGB stars caused by the interaction between Ti atoms and fullerenes?, The Astrophysical Journal Letters, 632 (2005) L159-L162.
Analysis of extraterrestrial minerals
Electron holography visualized vortex magnetism of ancient nano-magnetite
An isolated magnetite nanoparticle from a colloidal crystal in the Tagish Lake meteorite shows a concentric circular magnetic structure as displays here in a reconstructed phase distribution observed by an electron holography transmission electron microscope. We demonstrated existence of droplets as last moments of water in an ancient asteroid and the formation process of the framboids composed of three-dimensionally aligned nano-magnetite.
Reference
- Y. Kimura, T. Sato, N. Nakamura, J. Nozawa, T. Nakamura, K. Tsukamoto, K. Yamamoto, Vortex magnetic structure in framboidal magnetite reveals existence of water droplets in an ancient asteroid, Nature Communications, 4 (2013) 2649. doi: 10.1038/ncomms3649.
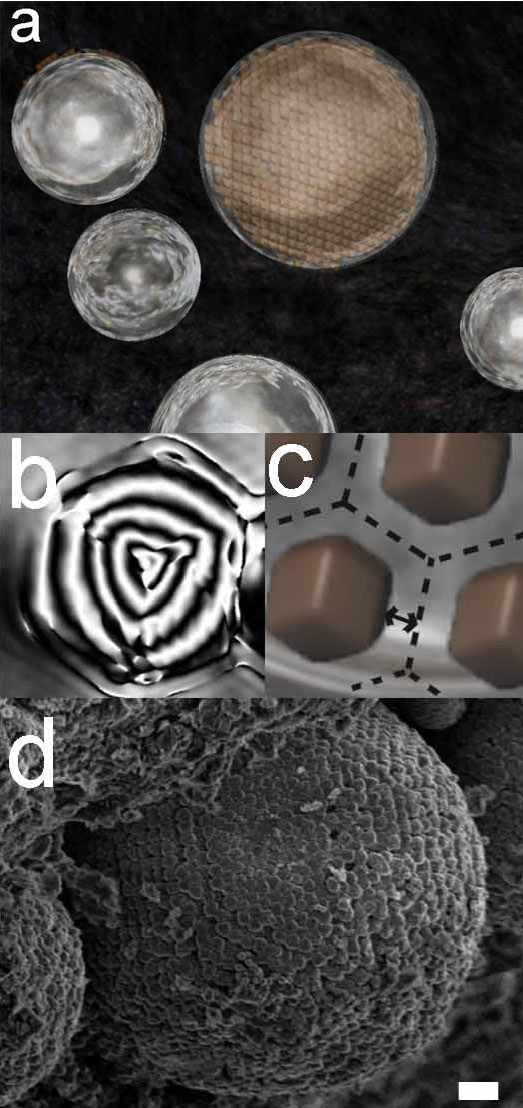
Surface nanotopography of matrix olivines
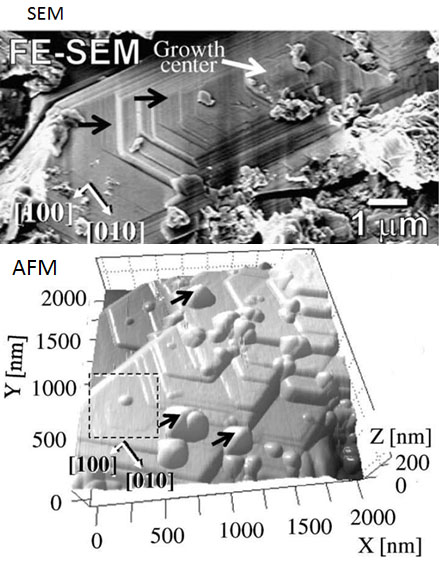
By means of nanoscale surface observation, we have proposed a new approach for investigating fine crystals of cosmic materials to reveal their origin and growth conditions. In the present work, molecular level topography of the faceted matrix olivine by Atomic Force Microscopy (AFM) has successfully been performed. The matrix olivine found to have preserved growth step pattern on its surface even though quite long time has passed since they formed in the early Solar System. The surface pattern suggests that the faceted matrix olivine could have been condensed from the gas phase, and possibly that these olivine crystals had continued to grow under a rapid cooling condition (0.1–1 K s).
Reference
- J. Nozawa, K. Tsukamoto, H. Kobatake, J. Yamada, H. Satoh, K. Nagashima, H. Miura, Y. Kimura, AFM study on surface nanotopography of matrix olivines in Allende carbonaceous chondrite, Icarus, 204 (2009) 681-686.
Production of carbyne crystal
Formation of carbine by SR irradiation
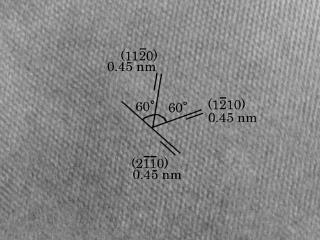
A new formation route of carbyne crystals using synchrotron radiation has been proposed. Formation of cosmic carbyne crystals has been discussed thermal process so far. In our experiment, carbyne crystalline film with micrometer in area was formed within 30 min by an irradiation of white synchrotron radiation beam. The total flux of irradiated photon was coincident with that for only 32 days at a distance of 5 AU from the young Sun. This result implies abundant production of carbyne crystals in the early solar nebula. Furthermore, graphitic carbon looks like pyrocarbons was produced for further irradiation. Generally, pyrocarbons founding in meteorites have been considered as a result of pyrolysis of hydrocarbons. This paper suggests an importance to consider non-thermal driving force to form cosmic dusts.
Reference
- Y. Kimura, C. Kaito, Possible Driving Force behind Formation of Cosmic Carbyne Crystals, The Astrophysical Journal Letters, 658 (2008) L83-86.
- Y. Kimura, C. Kaito, K. Hanamoto, M. Sasaki, S. Kimura, T. Nakada, Y. Saito, Y. Nakayama, Growth Process of Carbyne Crystal by Synchrotron Irradiation, Carbon, 40 (2002) 1043-1050.
Production of functional nanoparticles
ZnO nanoparticle
Crystal growth mechanisms in an electrical field have been discussed using zinc oxide nanoparticles. Produced ZnO nanoparticles were multiple twins in spite of strong iconicity and were spherical shape against its general tetrapod shape. Suppression of a growth of ZnO needle is an advantage for cosmetic material safely.
Reference
- C. Kaito, Y. Kinuta, H. Suzuki, S. Adachi, A. Kumamoto, Y. Saito, Y. Kimura, Morphological Alteration and Structure on ZnO Particles Produced in Electric Field, Journal of the Physical Society of Japan, 77 (2008) 094708-1 – 094708-4.




